Sep. 7, 2017 Cell: Oncolytic virus and c-Rel inhibitors make immunotherapy more effective
Immunotherapy is a promising approach in the treatment of cancer; but for most patients, immunotherapy drugs so far have failed to live up to their promise and provide little or no benefit. Immunotherapy combination might be the promising means of solving this problem, which could be supported by two studies published on September 7 in the journal Cell.
1. PD-1 antibody combining oncolytic virus with promising therapeutic effects
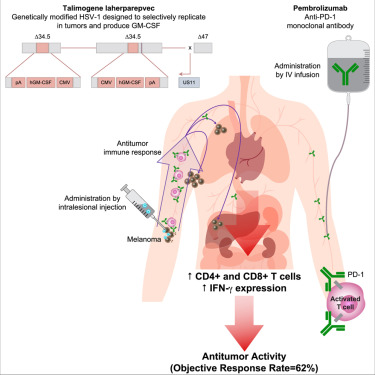
In a phase 1b clinical trial with 21 patients, researchers tested the safety and efficacy of combining the immunotherapy drug pembrolizumab, a checkpoint inhibitor, with an oncolytic virus called T-VEC. The results suggest that this combination treatment, which had a 62% response rate, may work better than using either therapy on its own. The study was published on September 7 in the journal Cell.
"We had a hypothesis about how these treatments would work together, and when we did biopsies of patients' tumors we found that they were cooperating in just the way we thought they would," says lead author Antoni Ribas, director of the Immunology Program at the UCLA Jonsson Comprehensive Cancer Center.
源自Cell
Make the “cold tumor” turns to be the “hot tumor””
"Some people put tumors into the categories of either 'hot' and 'cold,'" Ribas explains. "Hot tumors, also called inflamed tumors, have a lot of immune cells in and around them, but cold tumors do not." Drugs like pembrolizumab boost the response in tumors where immune cells are present but don't work in tumors where there is no immune response to boost.” This is where T-VEC comes in.
Oncolytic virus is also regarded as an immunotherapy. Oncolytic virus not only directly destroy tumor cells, but also stimulate anti-tumor immune response of the host. By injecting T-VEC into the patients' tumors, even those that were located deeper in the body, the researchers were able to transform cold tumors into hot ones, which in turn allowed pembrolizumab to deliver a beneficial enhancement. At the start of the trial, the patients were given two injections of T-VEC into their tumors three weeks apart. Starting at six weeks, they began receiving pembrolizumab every two weeks, at the same time as additional T-VEC injections. When the researchers took biopsies of the patients' tumors during the trial, they confirmed that their hypothesis about how the tumors would respond was correct. At six weeks, after two treatments with T-VEC but before the checkpoint drug was started, most tumors were infiltrated with T cells. At 30 weeks, the T cells remained in the area, but the majority of the tumor cells were gone.
More effective, no more worse side effects
The phase 1b multicenter trial included 21 patients on three continents, all of whom had metastatic melanoma. The researchers report that 62% had an overall response to the drug combination, meaning that their tumors decreased in size. One-third had a complete response, meaning that their tumors were undetectable. These responses are much higher than what would have been expected with either treatment alone—usually about 35%-40%.
More excitingly, the side effects in the study were no worse than what is observed when either drug is used on its own, and included fatigue chills, and fever. Three patients had more serious autoimmune side effects, which are sometimes seen after pembrolizumab treatment.
2. Immune checkpoint inhibitors work better when combined with drugs that increase blood flow
Cancer immunotherapy drugs only work for a minority of patients, but a generic drug now used to increase blood flow may be able to improve those odds, a study by Columbia University Medical Center (CUMC) researchers suggests.
源自Cell
In mice with melanoma, the researchers found that the drug - called pentoxifylline - boosts the effectiveness of immune-checkpoint inhibitors, a type of immunotherapy now commonly used in the treatment of melanoma and other cancers. The study was also published on September 7 in the online edition of Cell.

源自Immunity
"In advanced melanoma, for example, the cure rate is only about 20 percent. That's a remarkable improvement over previous therapies," says study leader Sankar Ghosh, PhD, Chair and Silverstein and Hutt Family Professor of Microbiology & Immunology. "But why doesn't it work for the other 80 percent? There must be another mechanism that contributes to the suppression of the immune response."
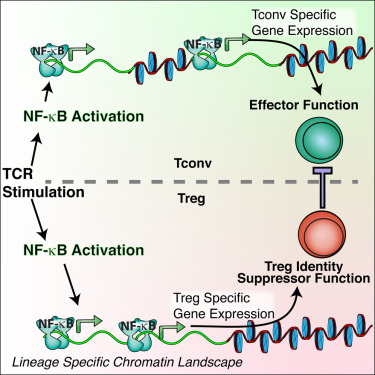
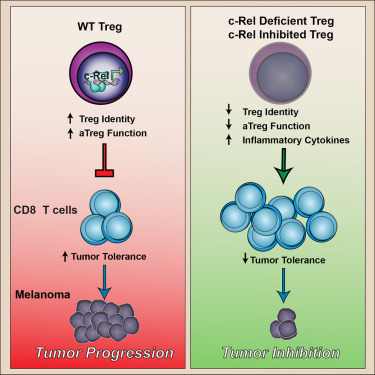
Dr. Ghosh and other cancer biologists suspected that a different type of T cell, known as regulatory T cells, or Tregs, may also suppress the immune system's attack on cancer. Large numbers of these cells are found within several types of tumors. "One possible therapy would be to get rid of Tregs," he said. "But Tregs are also needed to keep the immune system in check, and shutting down Tregs completely would unleash an attack against the body's healthy cells and organs."
源自Cell
This point is underscored by a related study, published on the same day in Immunity, in which Dr. Ghosh and colleagues found that removing NF-B from Tregs caused widespread and lethal autoimmunity in mice. However, a partial inhibition of NF-κB, achieved by removing only one, specific, NF-B protein, called c-Rel, changed Treg function without causing widespread autoimmunity. In the Cell study Ghosh and colleagues showed that these c-Rel deficient Tregs were specifically crippled in their ability to protect cancer cells. As a result, when c-Rel is blocked, killer T cells mounted a more robust attack on cancer cells without causing autoimmunity.
Pentoxifylline is a drug that is used in patients to increase blood flow in the hands and feet of people with poor circulation, but it's also known to inhibit the c-Rel protein. In the Cell study, the researchers demonstrated that pentoxifylline blocked Treg function and boosted the effectiveness of standard checkpoint-blockade immunotherapies. "The next step is to test this drug combination in human clinical trials," Dr. Ghosh says.
Reference:
1.http://www.biodiscover.com/news/research/725886.html
2. https://medicalxpress.com/news/2017-09-immunotherapy-combination-safe-percent-effective.html

